Clinical trials have demonstrated the effectiveness of TMS Therapy in treating patients who have not benefited from prior antidepressant medication. TMS Therapy was studied in adult patients suffering from Major Depressive Disorder, all of whom had not received satisfactory improvement with previous treatments.
An Effective and Durable Option for Treating Major Depressive Disorder
In an independent, randomized, controlled trial funded by the National Institute of Mental Health, 307 patients were treated with the NeuroStar TMS System for 4 to 6 weeks, similar to real clinical context.1
Patients were divided into two groups:
- Low Treatment Resistance: Patients who have failed to improve their depression symptoms after a single antidepressant treatment of adequate dose and duration.
- High Treatment Resistance: Patients who have failed to improve their depression symptoms after a multiple (2-14) antidepressant treatments of adequate dose and duration.
At the end of their treatments, patients who had received TMS Therapy were four times more likely to achieve remission compared to patients receiving a sham treatment. 1 in 2 patients experienced significant improvement in their depression symptoms and 1 in 3 experienced complete remission. Patients treated with the TMS System also experienced significant improvement in anxiety and physical symptoms (such as appetite changes, aches and pains, and lack of energy) associated with depression.1
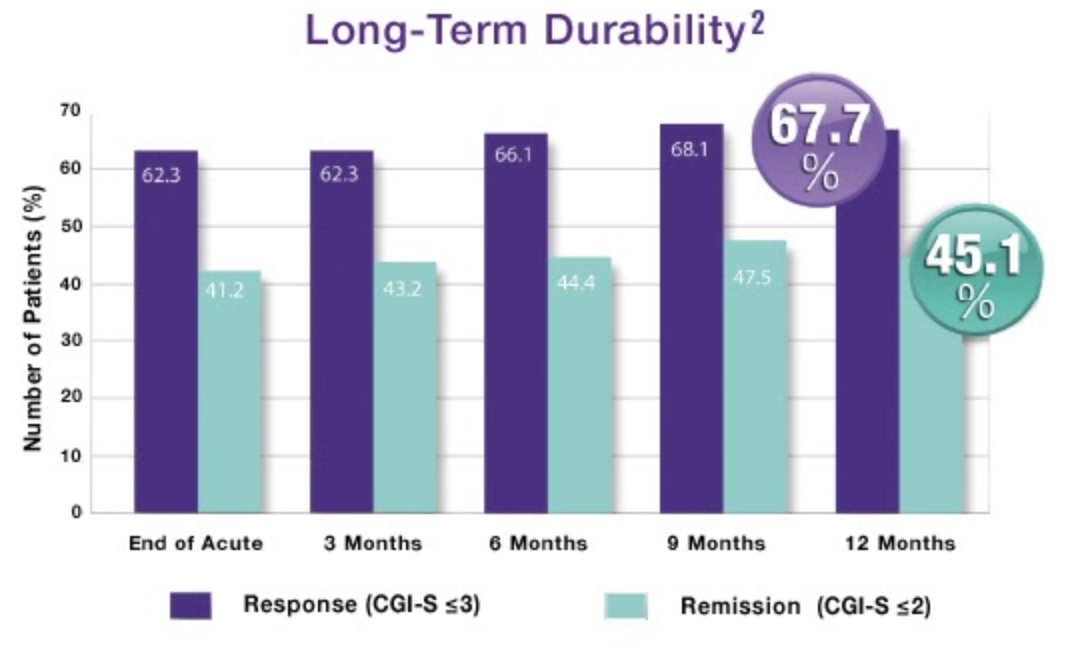
Durability of TMS Treatments
In a trial with physician directed standard of care, meaning TMS Therapy could be used in conjunction with antidepressants as needed, patients who had received treatment then reported their symptom levels at 3, 6, 9 and 12 months to determine the durability of their treatments. By the end of the 12-month period, 2 out of 3 patients who had either responded or completely remitted after TMS treatment remained at the symptom levels they reported at the end of the treatment phase.1
After the end of the treatment period, only 1 in 3 patients needed to come back for maintenance TMS sessions, or ‘reintroduction’ during this 12-month period.1
Treatment Algorithm
Additionally, GenHealth follows a specific tool to use in educating patients on the best practices of treating depression. The Best Practices Treatment Guideline for Depression has been developed to help patients understand TMS Therapy as an option if their first line antidepressant medications stop working. This guideline is based on the 2010 American Psychiatric Association’s practice guidelines and TMS Therapy indication for use, which says:
TMS Therapy is indicated for the treatment of major depressive disorder in adult patients who have failed to achieve satisfactory improvement from prior antidepressant medication at or above the minimal effective dose and duration in the current episode.
Please see the Treatment Algorithm for an effective illustration of the use of TMS Therapy early on in the treatment of depression.
TMS Therapy has not been studied in patients who have not received prior antidepressant treatment.
 TMS+YOU is an online community and national patient advocacy site for TMS Therapy. Those considering Transcranial Magnetic Stimulation can connect with patients who have had the treatment to answer questions, share insights, and get the latest information.
TMS+YOU is an online community and national patient advocacy site for TMS Therapy. Those considering Transcranial Magnetic Stimulation can connect with patients who have had the treatment to answer questions, share insights, and get the latest information.
References:
- Carpenter LL, et al. (2012). Depress Anxiety, 29(7):587-596.

